Beginning January 1, 2024, CMS is requiring all 340B covered entities that submit claims for separately payable Part B drugs and biologicals to report modifier “JG” or “TB” on claim lines for drugs acquired through the 340B Drug Pricing Program.
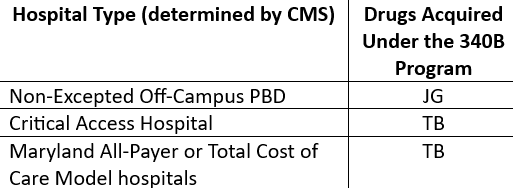
Starting January 1, 2024, the following provider types, who were historically exempt from reporting these modifiers, must begin reporting the “JG” or “TB” modifiers on separately payable claim lines (status indicator “K”) for drugs acquired through the 340B Program, as identified in the below table:
While these modifiers will not trigger a payment reduction, the “JG” and “TB” modifiers will be used for informational purposes to comply with certain requirements of the Inflation Reduction Act of 2022 (“Act”). The Act establishes a Part B inflation rebate by manufacturers for certain single source drugs and biologicals with prices that are increasing faster than the rate of inflation. Drug purchased on 340B are specifically excluded from the units of drugs for which a manufacturer otherwise may have a Part B inflation rebate liability. Reporting these modifiers allows CMS to identify drugs acquired through the 340B Program as part of implementing the Act, so that the 340B units can be subtracted from the total number of units for which a manufacturer otherwise may have a Part B inflation rebate liability.
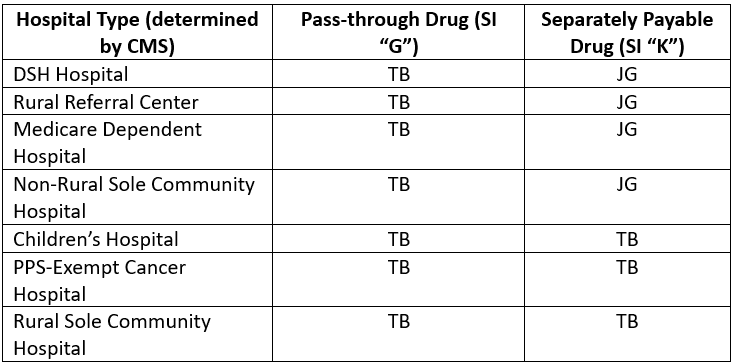
For all other 340B covered entity types, the following chart lists the modifiers hospitals paid under the Outpatient Prospective Payment System (“OPPS”) should report depending upon its hospital type and includes the pertinent OPPS drug status indicator for the 340B acquired drug being furnished:
For any questions regarding this updated policy and use of these modifiers, or for assistance in any other healthcare regulatory and operational matters for your organization, please contact Advis through our website or give us a call at 708-478-7030.
Published: November 2, 2023