On July 19, the Centers for Medicare & Medicaid Services (“CMS”) issued proposed rules for the Calendar Year (“CY”) 2022 Hospital Outpatient Prospective Payment System (“OPPS”) and Ambulatory Surgical Center (“ASC”) Payment System. Key proposals include: significantly increased civil monetary penalty (“CMP”) amounts for noncompliance with price transparency regulations; an increase in OPPS and ASC payment rates; a halt to the elimination of the Inpatient Only (“IPO”) list; removal of procedures from the ASC Covered Procedures List (“CPL”); and maintaining the status quo OPPS payment for 340B-acquired drugs.
Please note that these changes are merely proposed changes. They have not yet been finalized. Comments on the proposed rules must be submitted to CMS by September 17, 2021. A summary of the key points follows below.
Price Transparency Modifications
Over the last several years, a series of legislation and rulemaking has prescribed various requirements for hospitals to make public charges for items and services provided. CMS is now proposing:
- Increase in CMP amounts for noncompliance using a scaling factor based on hospital bed count – Concerned with the lack of hospital compliance with the price transparency rules, CMS is proposing the following scaled approach to determining a CMP amount (currently $300 per day):
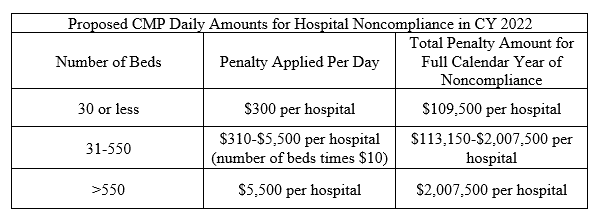
*In subsequent years, amounts adjusted according to 45 CFR § 180.90(c)(3).
CMS is seeking comment on the sliding scale approach, based on the hospital’s number of beds, to determine the CMP amount.*In subsequent years, amounts adjusted according to 45 CFR § 180.90(c)(3).
- Prohibition of certain conduct deemed a barrier to accessing standard charge information – CMS is proposing to amend price transparency regulations to specify that hospitals must ensure that the standard charge information is easily accessible, without barriers, including but not limited to, ensuring that the information is accessible to automated searches and direct file downloads through a link posted on a publicly available website. CMS commented that this additional requirement will ensure greater accessibility to the machine-readable file and its contents. It’s thought to prohibit practices noted in compliance reviews, such as lack of a link for downloading a single machine-readable file, using “blocking codes” or CAPTCHA, and requiring the user to agree to terms and conditions, or submit other further information, prior to access.
- Modification to deeming policy to include state forensic hospitals as having met the price transparency requirements, so long as the facility provides treatment exclusively to individuals who are in the custody of penal authorities and does not offer services to the general public.
- CMS is seeking clarifications and requests for comment on other various items to improve standardization of data disclosed by hospitals, including:
- Consideration related to price estimator tool policies – identifying best practices, common features, and solutions to overcoming common technical barriers.
- Whether there should be a requirement for specific “plain language” standards in describing shoppable services.
- Methods to identify and highlight exemplary hospitals.
- Improving standardization of machine-readable files.
OPPS and ASC Payment Increase
CMS proposed to increase payment rates under the OPPS by 2.3 percent. CMS proposed to continue to implement the statutory 2 percent point reduction in payments for hospitals that fail to meet outpatient quality reporting requirements. Similarly, using the hospital market basket methodology, for CY 2022, CMS proposed to increase payment rates under the ASC payment system by 2.3 percent for ASCs that meet the quality reporting requirements under the Ambulatory Surgical Center Quality Reporting (“ASCQR”) Program.
Due to the impact of COVID-19 on outpatient service utilization, CMS is proposing to use CY 2019 (instead of CY 2020) data to set CY 2022 OPPS and ASC payment rates.
Backtracking on Elimination of IPO List
The IPO list identifies services for which Medicare will only make payment when the services are furnished in the inpatient hospital setting because of the nature of the procedure, the underlying physical condition of the patient, or the need for at least 24 hours of post-operative recovery time or monitoring before the patient can be safely discharged. In the CY 2021 OPPS/ASC final rule, CMS finalized a policy to eliminate the IPO list over a three-year period. As part of the first phase, CMS removed 298 codes from the list beginning in CY 2021.
CMS is now proposing to halt the elimination of the IPO list. After clinical review of the 298 services removed from the list in CY 2021, CMS proposes to add those 298 services back to the IPO list beginning in CY 2022. CMS further proposes to codify the five longstanding criteria for determining whether a service or procedure should be removed from the IPO list. CMS will assess annually whether a service or procedure on the IPO list should be removed from the list by determining whether the service or procedure meets at least one of the following criteria:
- Most outpatient departments are equipped to provide the service or procedure to the Medicare population.
- The simplest service or procedure described by the code may be performed in most outpatient departments.
- The service or procedure is related to codes that CMS has already removed from the IPO list.
- CMS determines that the service or procedure is being performed in numerous hospitals on an outpatient basis.
- CMS determines that the service or procedure can be appropriately and safely performed in an ASC, and is specified as a covered ASC procedure, or CMS has proposed to specify it as a covered ASC procedure.
CMS proposes to exempt procedures that are removed from the IPO list under the OPPS beginning on or after January 1, 2021, from site-of-service claim denials, Beneficiary and Family-Centered Care Quality Improvement Organization (“BFCC-QIO”) referrals to Recovery Audit Contractor (“RAC) for persistent noncompliance with the 2-midnight rule, and RAC reviews for “patient status” (site-of-service) for a time period of two years (vs. indefinitely exempted as set out in the CY 2021 OPPS/ASC final rule).
Removing Procedures from ASC CPL
CMS is proposing to re-adopt the ASC CPL criteria that were in effect in CY 2020 and to remove 258 of the 267 procedures that were added to the ASC CPL in CY 2021. CMS is also proposing to change the notification process adopted in CY 2021 to a nomination process that would allow stakeholders to nominate procedures they believe meet the requirements to be added to the ASC CPL. This new nomination process would begin in CY 2023.
OPPS Payment for 340B-Acquired Drugs Remains the Same
CMS proposes to continue its current policy of paying an adjusted amount of ASP minus 22.5 percent for drugs and biologicals acquired under the 340B program. CMS further proposes to continue to exempt Rural SCHs, PPS-exempt cancer hospitals and children’s hospitals from this 340B payment policy.
Solicitation for Comments on Temporary Policies Implemented During COVID-19
Many regulatory flexibilities implemented during COVID-19 will expire at the end of the public health emergency. CMS is seeking comment on whether there are certain policies that should be made permanent, specifically services furnished by hospital staff to beneficiaries in their homes through the use of communication technology, direct supervision when the supervising practitioner is available through two-way, audio/video communication technology, and code and payment for COVID-19 specimen collection.
Rural Emergency Hospital (“REH”) Provider Type – Request for Information
Section 125 of the Consolidated Appropriations Act (“CAA”) of 2021 established REHs as a new provider type. The legislation provides a mechanism for Critical Access Hospitals (“CAHs”) and certain rural hospitals to convert to the REH provider type, effective January 1, 2023. REH services specifically identified in the legislation include emergency department services and observation care (no acute care inpatient services may be provided), with payment at the OPPS rate plus five percent. There is also an additional facility payment as determined by a statutory formula.
CMS is seeking public comment via a Request for Information on the health and safety standards, payment policies, the REH enrollment process, and quality measures and reporting requirements to REHs to inform future policy making.
Updates to the Hospital Outpatient Quality Reporting (“OQR”) Program
CMS is proposing changes for the CY 2023, CY 2024, CY 2025, and CY 2026 payment determinations and subsequent years, specifically:
- Remove the OP-02: Fibrinolytic Therapy Received Within 30 Minutes of ED Arrival measure, beginning with the CY 2025 payment determination.
- Remove the OP-03: Median Time to Transfer to Another Facility for Acute Coronary Intervention measure, beginning with the CY 2025 payment determination.
- Adopt the COVID-19 Vaccination Coverage Among Health Care Personnel (“HCP”) measure, beginning with the CY 2024 payment determination.
- Adopt the Breast Screening Recall Rates measure, beginning with the CY 2023 payment determination.
- Adopt the ST-Segment Elevation Myocardial Infarction (“STEMI”) electronic clinical quality measure (“eCQM”), beginning with voluntary reporting for the CY 2023 reporting period and mandatory reporting beginning with the CY 2024 reporting period/CY 2026 payment determination.
- Make voluntary the reporting of the OP-37a-e: Outpatient and Ambulatory Surgery Consumer Assessment of Healthcare Providers and Systems (“OAS CAHPS”) Survey-based measures, beginning with the CY 2023 reporting period and mandatory beginning with the CY 2024 reporting period/CY 2026 payment determination.
- Make mandatory the reporting of the OP-31: Cataracts: Improvement in Patient’s Visual Function within 90 Days Following Cataract Surgery measure, beginning with the CY 2025 payment determination.
Beginning with the CY 2024 payment determination, CMS is also proposing three updates to its validation requirements by proposing to:
- Use electronic file submissions for chart-abstracted measure medical record requests.
- Change the chart validation requirements and methods.
- Update the targeting criteria.
Updates to the ASCQR Program
CMS is proposing changes for CY 2024, CY 2025, and CY 2026 payment determinations and subsequent years. Specifically, CMS is proposing to:
- Adopt the COVID-19 Vaccination Coverage Among HCP measure, beginning with the CY 2024 payment determination.
- Resume data collection for four measures, beginning with the CY 2025 payment determination.
- ASC-1: Patient Burn;
- ASC-2: Patient Fall;
- ASC-3: Wrong Site, Wrong Side, Wrong Patient, Wrong Procedure, Wrong Implant; and
- ASC-4: All-Cause Hospital Transfer/Admission.
- Require the ASC-11: Cataracts: Improvement in Patient’s Visual Function within 90 Days Following Cataract Surgery measure, beginning with the CY 2025 payment determination.
- Require the ASC-15a-e: OAS CAHPS Survey-based measures with voluntary reporting, beginning with the CY 2023 reporting period and mandatory reporting beginning with the CY 2024 reporting period/CY 2026 payment determination.
Radiation Oncology (“RO”) Model Updates
The RO Model will test whether prospective episode-based payments for radiotherapy (“RT”) services will reduce Medicare program expenditures and preserve or enhance quality of care for beneficiaries. Medicare would pay participating providers and suppliers a site-neutral, episode-based payment for specified professional and technical RT services furnished during a 90-day episode to Medicare fee-for-service beneficiaries diagnosed with certain cancer types. In keeping with congressional action, CMS is proposing to modify the model performance period to begin on January 1, 2022, and end on December 31, 2026. CMS is also proposing a number of other modifications to the RO Model’s timing and design.
Advis is available to assist providers in preparing comments for submission to CMS.
For any questions regarding the CY 2022 OPPS/ASC proposed rules, or for organizational assistance with any other healthcare regulatory or operational matters, please contact Advis at 708-478-7030.
Published: July 22, 2021





