340B ADR Proposed Rule – 2022
On November 29, 2022, The Department of Health and Human Services (HHS) issued a replacement proposed rule revising the 2020 final rule establishing the 340B Administrative Dispute Resolution (ADR) process.
HHS noted that Health Resources and Services Administration (HRSA) encountered “policy and operational challenges” while attempting to implement the 2020 final rule. Specifically, the HHS acknowledged that the 2020 final rule caused accessibility issues for covered entities that are small, community-based organizations with limited means. HHS further acknowledged that the 2020 final rule did not require 340B ADR Panel members to process 340B specific knowledge. Instead, the 2020 final rule only required that the 340B ADR Panel possess relevant expertise and experience in drug pricing or drug distribution and/or expertise in handling complex litigation. HRSA acknowledged that the 340B ADR Panel must be required to possess specialized 340B related expertise given the unique and complex nature of the 340B Statute and Program framework.
The revised proposed rule will streamline the dispute resolution process for covered entities and ensure that the process is efficient, effective, and timely. The proposed rule also more closely aligns the ADR process with the 340B Statute. The rule simplifies the ADR filing process and provides further clarity regarding the adjudication process. Additionally, the revised proposed rule implements a revised structure of the ADR Panel with additional screening safeguards to prevent conflicts of interest and ensure that OPA employees selected to ADR Panels possess specific 340B subject matter knowledge. Additionally, the 2022 proposed rule includes a reconsideration process for parties dissatisfied with a 340B ADR Panel’s decision.
Pursuant to the 2022 proposed rule, any claims that are in process and have been submitted pursuant to the 2020 final rule would be automatically transferred to the new process.
Below is a summary of the key highlights from the 2022 proposed rule:
ADR Panel Qualifications and Screening Procedures
- HHS proposes that the Secretary appoint a roster of eligible individuals, consisting of OPA staff, to serve on 340B ADR Panels. The roster will include no less than 10 OPA staff members. Additionally, the OPA director will select at least three members from the roster to form a 340B ADR Panel to facilitate the review and resolution of an ADR claim. CMS representatives will not be included on this roster.
- Dedicated OPA staff members will have specific ADR duties as a part of their job functions, including being a part of the 340B ADR Panel that makes decisions on an ADR claim.
Duties of the ADR Panel
- The ADR Panel will independently review and apply 340B law to “case-specific” factual circumstances.
- The ADR Panel will review and evaluate all documents and other evidence submitted by the party initiating the claim. The Panel may then request additional information or clarification from any involved party.
- If the Panel finds that either party did not fully respond to the Panel’s request for information, the Panel may draw an “adverse inference” and make a decision on the claim based on the information provided by parties.
Procedure for Filing Claims
- Covered entities and manufacturers must file a written claim to the OPA within 3 years of the alleged violation of the 340B statute. The 3-year limitation is consistent with 3-year the record retention requirement for entities participating in the 340B program.
- All parties to the claim must maintain all files, documents, or records regarding the specified claim until the final agency decision is issued.
Covered Entity Claims
- A claim filed by a covered entity must provide the basis for the covered entity’s belief that it has been overcharged by a manufacturer to qualify for the ADR process.
- Covered entities are required to provide documentation to prove accuracy of the claim. Accepted documentation includes but is not limited to: (1) a 340B purchasing account invoice which shows the purchase price by national drug code, less any taxes or fees; (2) the 340B ceiling price for the drug during the quarter(s) corresponding to the time period(s) of the claim; (3) documentation by the manufacturer or wholesaler of the attempts made to purchase the drug via a 340B account at the ceiling price, which resulted in the instance of alleged overcharging; (4) documentation with HRSA regarding the alleged overcharge, including price unavailability forms or other correspondence; and (5) an estimate of the monetary charges.
- Covered entities will also be required to provide OPA with a written summary of attempts to work in good faith to resolve the instances of overcharging with the manufacturer.
Manufacturer Claims
- A claim filed by a manufacturer must include documentation to support the claim that a covered entity violated the prohibition on diversion or duplicate discount.
- Manufacturers are required to provide documentation to prove accuracy of the claim. Accepted documentation includes but is not limited to: (1) a final audit report which indicates that the manufacturer audited the covered entity for compliance with the prohibition on diversion; (2) any communication with the State Medicaid agency indicating rebates claimed (for duplicate discount violations only); (3) the covered entity’s written response to the manufacturer’s audit finding(s); and (4) an estimate of monetary damages.
Combining Claims
- HHS proposed that covered entities and manufacturers be permitted to combine individual claims. For covered entity joint claims, the claim must list each covered entity and the corresponding 340B IDs. The covered entities must also include documentation demonstrating that each covered entity meets all the requirements for filing an ADR claim.
- A letter requesting combining of claims must company the joint claim at the time of filing. The letter must document that each covered entity consents to the combination of claims.
Claim Filing and Adjudication Process
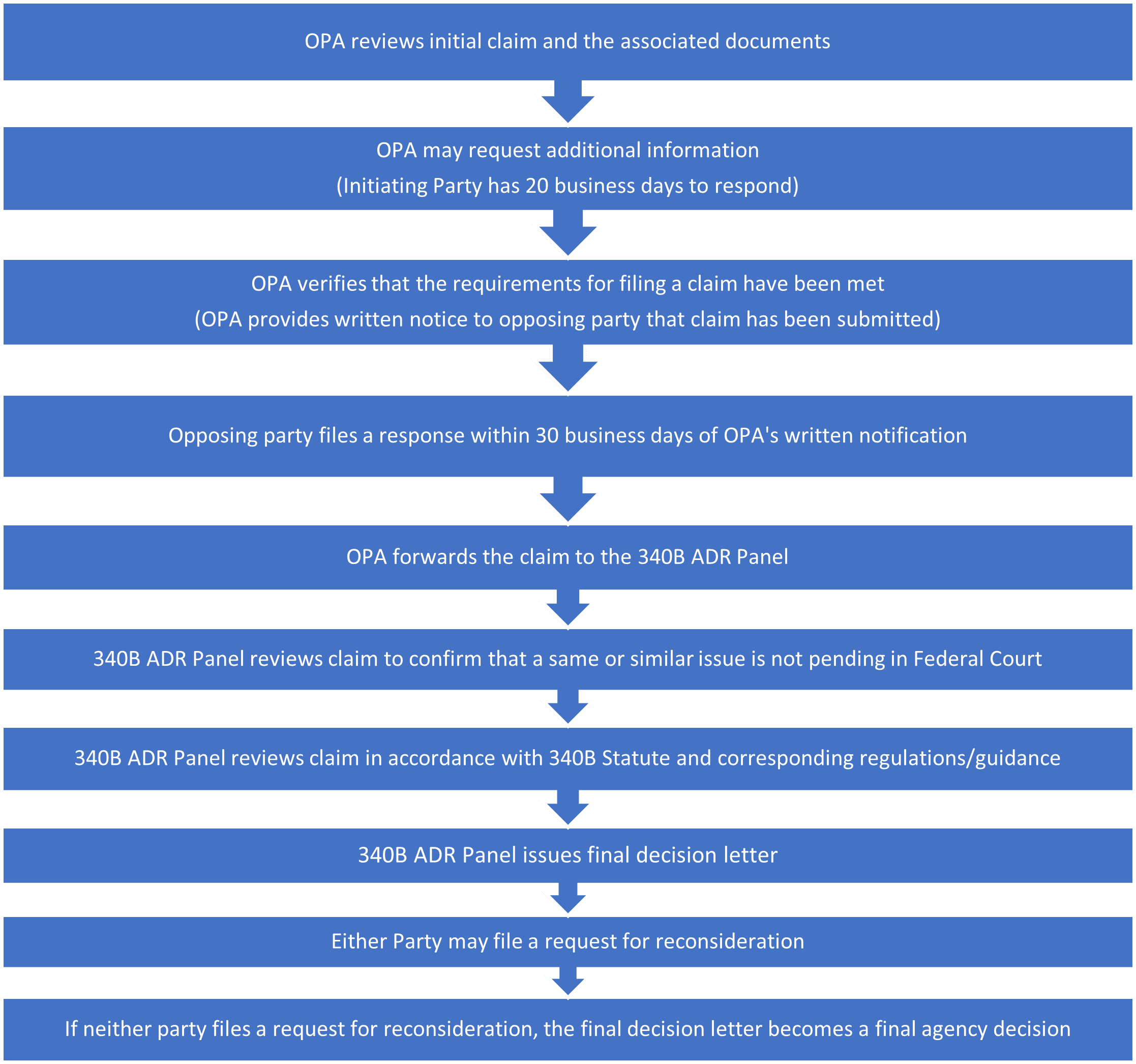
Discovery Procedures
- The Secretary for the 340B ADR process will establish procedures by which a covered entity may discover or obtain information and documents from manufacturers and third parties relevant to the to the specific claim.
- Such document requests will be facilitated via the 340B ADR Panel. Specifically, the covered entity must submit a written request to the 340B ADR Panel no later than 20 business days after the entity was notified by the OPA that the claim has moved forward for 340B ADR Panel review.
- If the 340B ADR Panel deems the document request to be “reasonable, relevant, and within the scope of the asserted claim,” it will notify the covered entity in writing.
- Once the 340B ADR Panel issues the document request to the manufacturer in question, the manufacturer must submit the requested documentation by a deadline specified by the 340B ADR Panel. Manufacturers may seek one extension to the proposed deadline by submitting a request to the 340B ADR Panel.
The 2022 proposed rule establishes a more accessible ADR Process that is efficient and timely for covered entities. The proposed rule streamlines and simplifies the filing and adjudication process. Advis is continuing to monitor new developments regarding the ADR Process. Interested covered entities should reach out to the Advis 340B experts at 708-478-7094.
Published: December 6, 2022





